Is diethyl ether good for extraction?
Why is diethyl ether used in extractions
Diethyl ether is a common laboratory solvent. It has limited solubility in water, thus it is commonly used for liquid-liquid extraction. Being less dense than water, the ether layer is usually on top.
What is the best solvent for extracts
Hexane – Hexane is a popular solvent for extraction as it has a very low VOC, is safe to be used with goods produced for consumption, and it produces no foul odor or poisonous fumes. All of this coupled with hexane's low boiling point, it is considered a safe, fast, easy to use solvent for extraction.
What is the use of ether in extraction
As an extraction medium, it is used to extract acetic acid and organic acids from aqueous systems in the cellulose acetate and plastics industry.
What solvents are used for extraction
Ether oil and n-hexane are the most common solvents. The solvent extraction method recovers almost all oils and only 0.5%–0.7% of oil remains in the seed. The solvent extraction method can be used for any low-oil material.
Which ethers are widely used as extraction solvent
Ethyl ether is an excellent solvent for extractions and for a wide variety of chemical reactions. It is also used as a volatile starting fluid for diesel engines and gasoline engines in cold weather. Dimethyl ether is used as a spray propellant and refrigerant.
Why is diethyl ether rather than acetone chosen as the extraction solvent
Diethyl ether is a relatively organic substance that can easily dissolve trimyristin more so than aqueous solvent. However, acetone is an organic solvent that can more easily dissolve trimyristin at a higher temperature than diethyl ether.
What five compounds are good extracting solvents
including water, absolute methanol, ethanol, acetone (the polarity indexes are 10.2, 5.1, 4.3, and 5.1, resp.), 50% methanol, 50% ethanol, and 50% acetone.
What is the best solvent for extracting cannabinoids
Ethanol
Ethanol (Ethyl Alcohol)
Denatured Alcohol 190 Proof 3C is an ethanol-based product available at Alliance Chemical that can be used for cannabis extraction. Ethanol is a popular and safe solvent, often used for its effectiveness in extracting a wide range of cannabinoids and terpenes.
Is ether a good extraction solvent
The ability of ethers to accept H-bonds combined with the London forces of the alkyl groups bonded to the oxygen allows ethers to be excellent solvents for a wide range of organic compounds. The low chemical reactivity of ethers also makes ethers a preferred solvent for many organic reactions.
Which ether is used as extraction solvent
The most common pair of extraction solvents used is diethyl ether (often referred to as simply 'ether') and water. Polarity is a relative term – ether is considered nonpolar and water polar. The fact that two phases are observed upon adding one to the other is a consequence of their different polarities.
What is the major disadvantage of using ether as an extraction solvent
Answer and Explanation:
Some disadvantages of using ether as an extraction solvent are : Ether is anesthetic and can induce sleep. Ether is flammable and can catch fire. Ether can easily oxidize into explosives.
Is diethyl ether a common solvent
Ether (diethyl ether, ethyl ether) is an extremely common solvent that is routinely used in varying quantity in laboratory procedures through out the University.
Why is diethyl ether a bad solvent
Solvents with very low boiling points (e.g. diethyl ether, acetone, and low-boiling petroleum ether) are highly flammable and can be difficult to work with as they readily evaporate. They can still be used with care, but if alternatives exist, they are often preferable.
What is the major disadvantage of using ether as an extracting solvent
Answer and Explanation:
Some disadvantages of using ether as an extraction solvent are : Ether is anesthetic and can induce sleep. Ether is flammable and can catch fire. Ether can easily oxidize into explosives.
Which pairs of solvents would make good extraction systems
Water and dichloromethane and water and diethyl ether form immiscible two-component solvent systems that are ideal for solvent extraction.
What is the best solvent for extracting terpenes
Non-volatile terpenes can be extracted using a very nonpolar organic solvent such as hexane. Using silica as a stationary phase in chromatography is another perfect tool to separate these terpenes from other compounds in the extract.
What are the disadvantages of diethyl ether
High exposure can cause unconsciousness and even death. * High exposure may affect the kidneys. * Repeated or prolonged skin contact can cause drying, scaling and cracking of the skin. * Diethyl Ether is a HIGHLY FLAMMABLE LIQUID and a DANGEROUS FIRE HAZARD.
What are the disadvantages of ether as an extraction solvent
Some disadvantages of using ether as an extraction solvent are :Ether is anesthetic and can induce sleep.Ether is flammable and can catch fire.Ether can easily oxidize into explosives.Ether vapor is quite dense.
What are the advantages disadvantages of using diethyl ether as an extraction solvent
Advantages and disadvantages of the use of ether:
| Advantages | Disadvantages |
|---|---|
| Its low boiling point keeps extraction temperatures low, which is especially useful for heat-sensitive compounds. | Because Ether is an anaesthetic, chemists in laboratories may experience nausea and unconsciousness while working with it. |
What is the problem with diethyl ether
* Breathing Diethyl Ether can cause drowsiness, excitement, dizziness, vomiting, irregular breathing, and increased saliva. High exposure can cause unconsciousness and even death. * High exposure may affect the kidneys. * Repeated or prolonged skin contact can cause drying, scaling and cracking of the skin.
Is acetone and diethyl ether a good extraction system
The combinations of ether and dichloromethane, water and ethanol, and acetone and diethyl ether are unsuitable for use as solvent extraction systems as there are insufficient differences in polarity to create the needed immiscibility.
What is the best solvent for cannabinoid extraction
sativa found that the hexane and ethanol mixture at 7:3 (v/v) is more efficient in extracting terpenes compared to hexane or ethanol alone [67]. Fischedick et al. [66] used ethanol as the extraction solvent to recover both terpenes and cannabinoids, and 36 compounds were identified in 11 varieties of C.
Is diethyl ether a safe solvent
High exposure can cause unconsciousness and even death. * High exposure may affect the kidneys. * Repeated or prolonged skin contact can cause drying, scaling and cracking of the skin. * Diethyl Ether is a HIGHLY FLAMMABLE LIQUID and a DANGEROUS FIRE HAZARD.
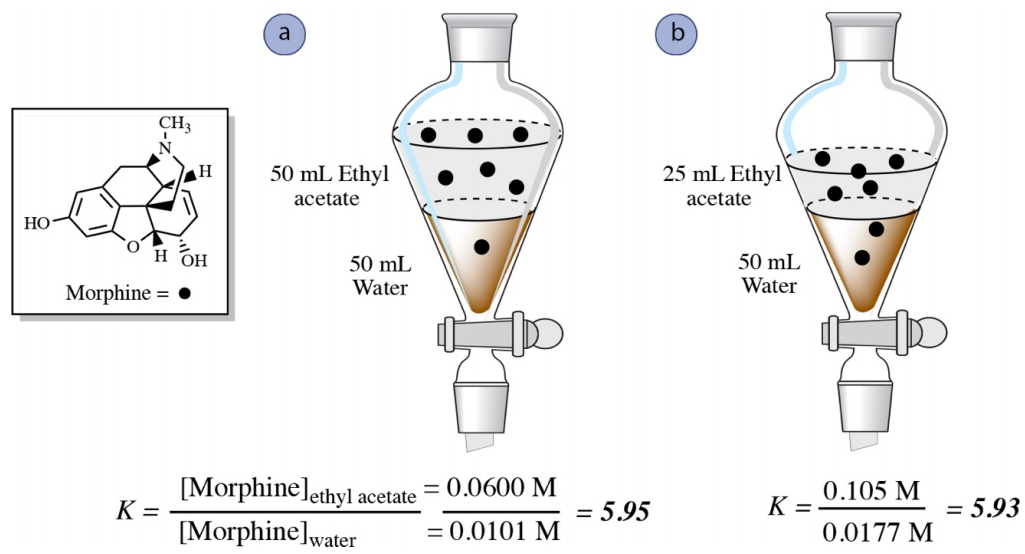
Which type of extraction is more efficient
multiple extractions
As a general rule, multiple extractions with small quantities of solvent or solution are more efficient than one extraction using the same amount of solvent (see below).
Is diethyl ether highly toxic
Toxicity. Acute: harmful by inhalation in high concentrations which can cause inebriation, sedation, unconsciousness and respiratory paralysis. Diethyl ether is irritating to the eyes, respiratory system and skin but these effects are usually reversible on removal of exposure.



0 Comments